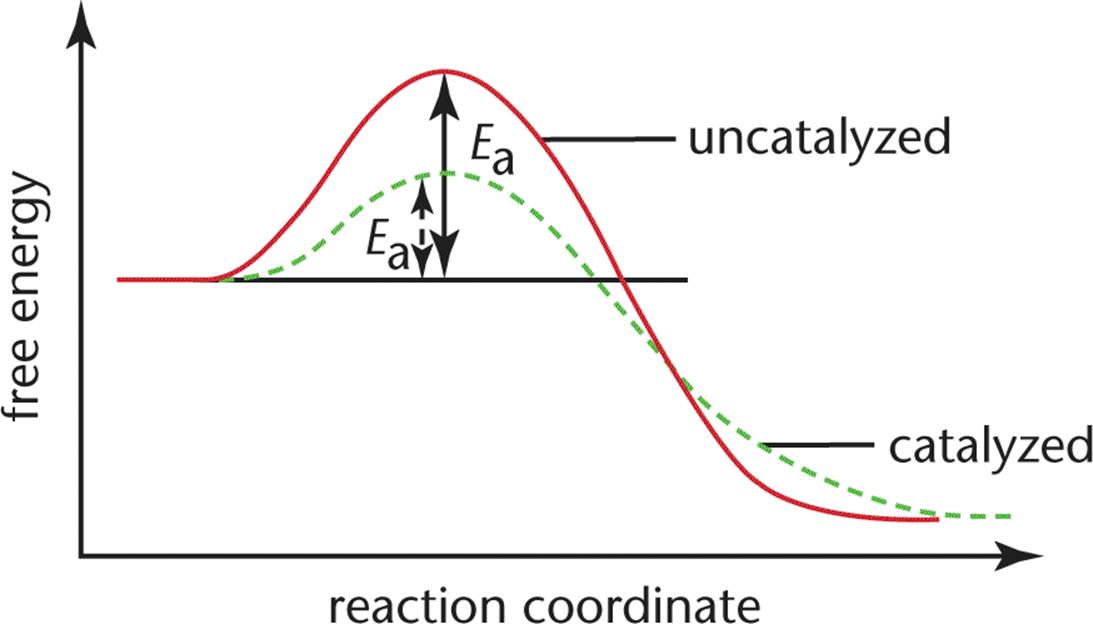
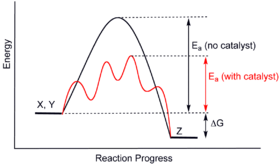
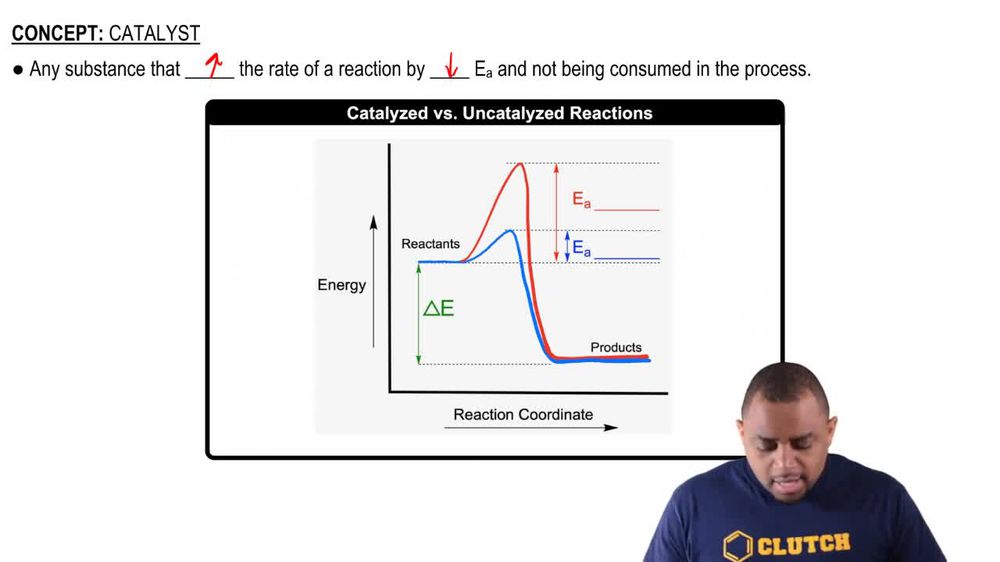
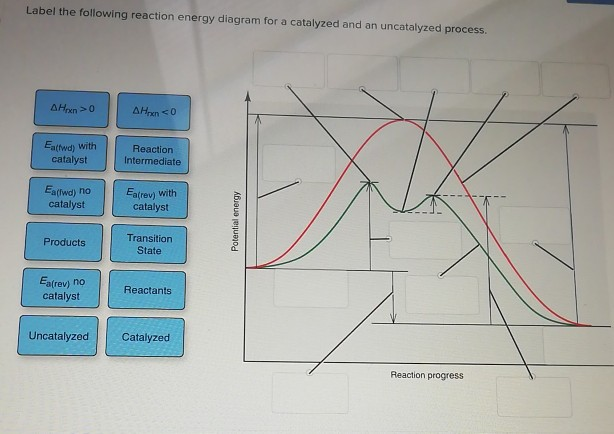
43 reaction energy diagram for a catalyzed and an uncatalyzed process
Reaction Kinetics: Reaction Mechanisms: Chemical Mechanisms - SparkNotes We will derive the Arrhenius Equation, which relates the rate constant for a reaction to its activation energy. Local minima on the reaction coordinate diagram are positions occupied by intermediates. By comparing the reaction coordinate diagram for a catalyzed and a uncatalyzed process, we can see that catalysts function by altering the route ... The Rate Of Reaction Of An Uncatalyzed And Catalyzed Reaction Have complex- the at the however for energy a reaction corresponding of reactant uncatalyzed reaction reactions lower higher mechanics same rate catalysis of en
Solved Label the following reaction energy diagram for a - Chegg Question: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. A > 0 Eatwa) no catalyst Exter, with catalyst Reactants Esinev) no Eastw with catalyst Potential energy Mono Products Uncatalyzed Catalyzed Transition State Reaction Intermediate Reaction progress Reset Zoom, This problem has been solved!

Reaction energy diagram for a catalyzed and an uncatalyzed process
Solved Label the following reaction energy diagram for a | Chegg.com Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. AHpXn > 0 Ealfwd) no catalyst Earrev) with catalyst Reactants Earrev) no catalyst Eaſfwd) with catalyst Potential energy AHpxn < 0 Products Uncatalyzed Catalyzed Transition State Reaction Intermediate Reaction progress Reset Zoom, Energy diagrams for enzyme‐catalyzed reactions: Concepts and ... The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. The survey of several Biochemistry textbooks reveals a high diversity of profiles for the same process. A, C, and D are adapted from Refs. 5, 3, and 2, respectively. Solved Label the following reaction energy diagram for a - Chegg Best Answer. 88% (42 ratings) Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
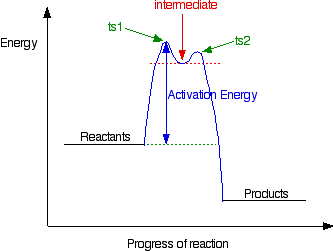
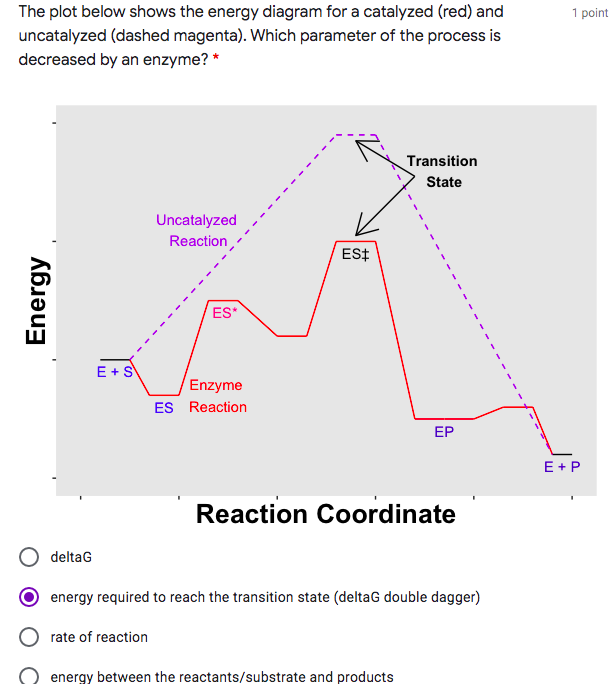
Reaction energy diagram for a catalyzed and an uncatalyzed process. What is the difference between a catalyzed and uncatalyzed reaction ... The uncatalyzed reaction proceeds via a one-step mechanism (one transition state observed), whereas the catalyzed reaction follows a two-step mechanism (two transition states observed) with a notably lesser activation energy. What is the activation energy for the uncatalyzed reaction What is the activation energy for the catalyzed reaction? SOLVED:uncatalyzed pathway for a reaction Skelch The energy diagram ... The following potential energy diagram shows they catalyzed in an uncapped allies path. To determine the catalyst path. It's going to be the one that has the lower activation energy, so it'll be the one that has the smaller peaks, the height of the peak corresponding to the magnitude of the activation energy mean. So the blue one will be the catalyst process because there are three peaks ... PDF Reaction Rates and Catalysts in Ethanol Production (One Activity) Students will be able to interpret and draw reaction progress diagrams for , catalyzed and uncatalyzed reactions. , Time Allotted , Five 45 minute periods are needed to complete the entire module. Any of the days can be combined to accommodate block scheduling. , Reaction energy diagram of a catalyzed and an | Chegg.com Reaction energy diagram of a catalyzed and an uncatalyzed process Which statement about catalyst is true? Molecules cannot react with each other unless a catalyst is present. A catalyst decreases the activation energy of both the forward and the reverse reaction. A catalyst is consumed during a reaction.
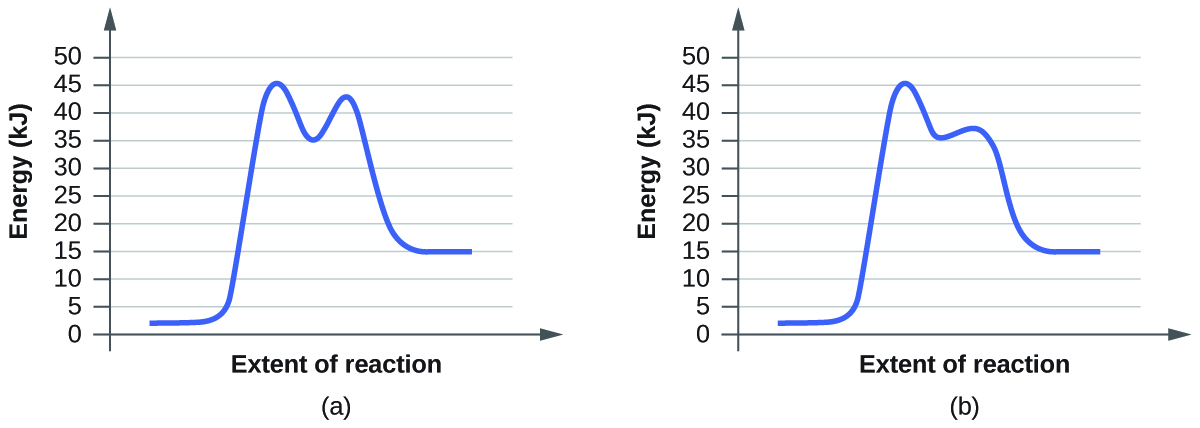
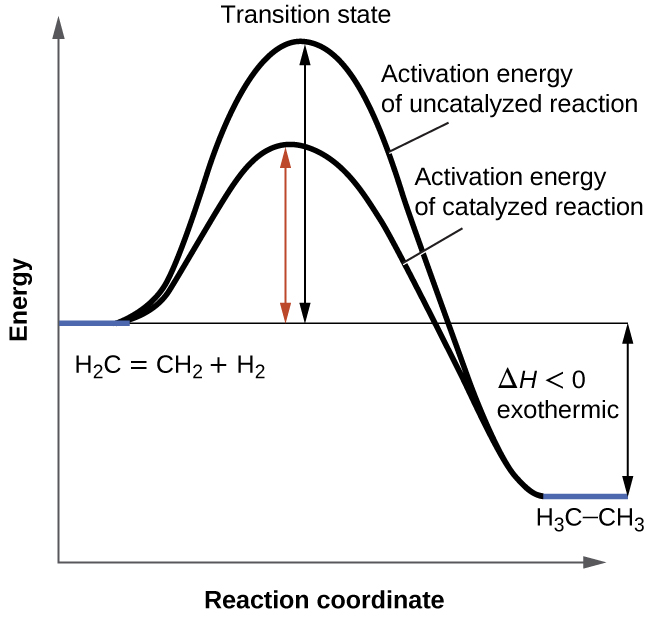
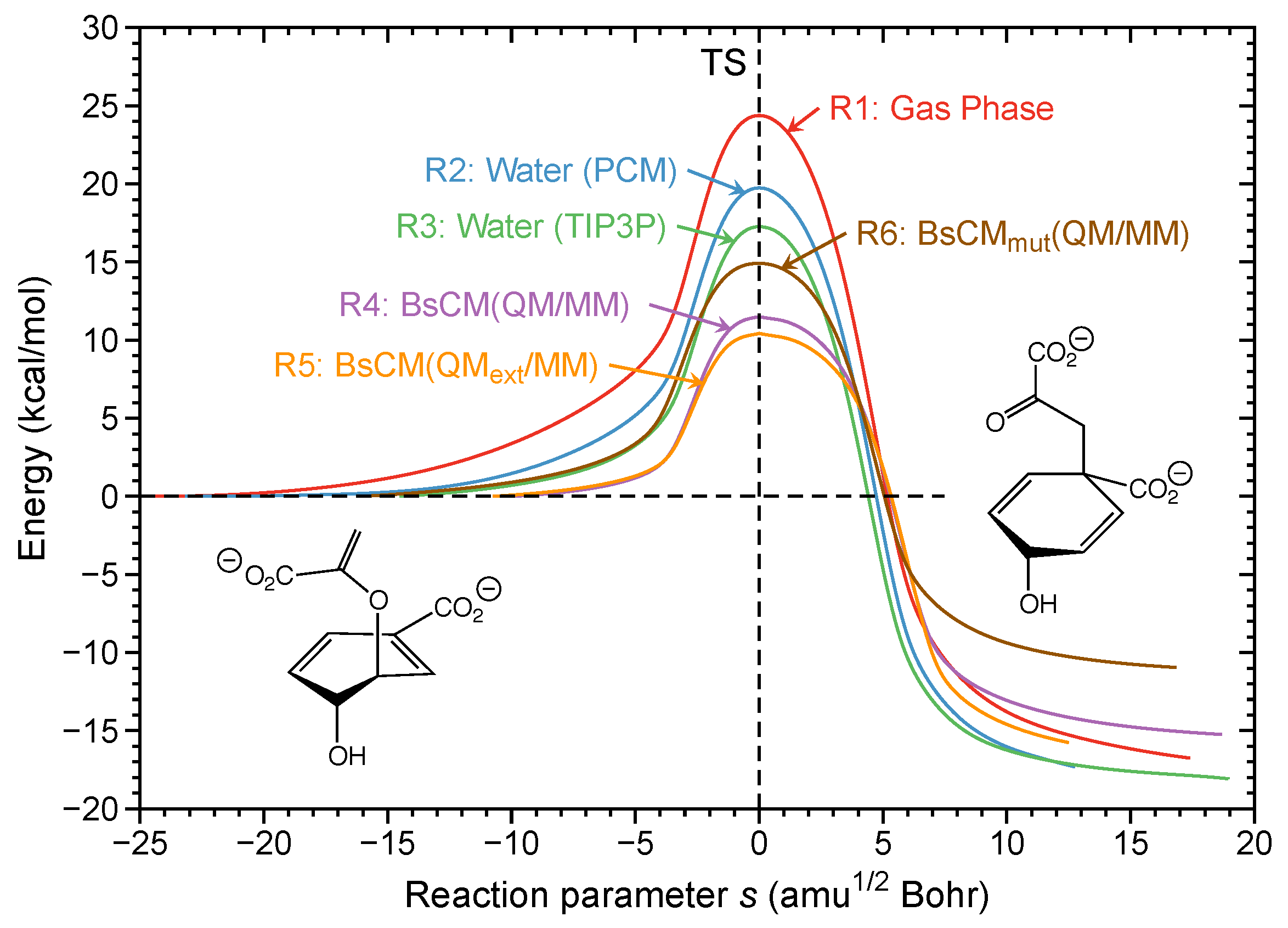
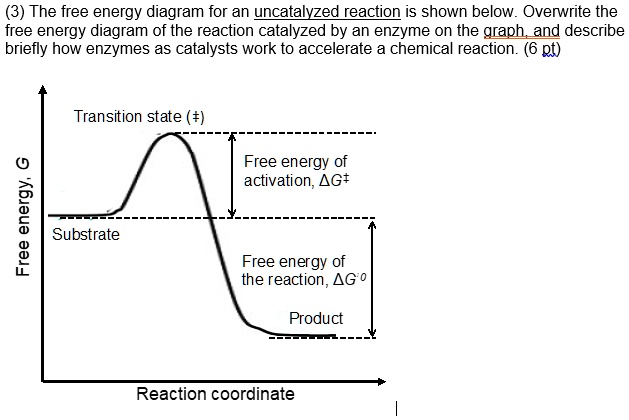
PDF Graph Review - ISD 622 Below is an Incomplete energy diagram. Activated Complex — 50 Reactants a. b. —10 kJ/m01. Label Use the following data to complete the diagram: Ea = +50 kJ/mol; AE forward the reactants, products, AE, Ea E' and the activated complex. \That is the value of E' ? (-00 3. Reaction coordinate diagram enzyme - Big Chemical Encyclopedia Computed three-dimensional free energy diagram for proline racemase x-axis, the reaction coordinate y-axis, free energy and z-axis, substrate saturation. At the front of the diagram, [S] = [P] = 1 /aM, where the enzyme is unsaturated at the back of the diagram, [S] = [P] = 1 M, where the enzyme is oversaturated. enzyme catalyzed reaction diagram - The Solutions Unit Energy diagram of enzyme-catalyzed and uncatalyzed reactions, from Wikipedia The peak of this energy diagram represents the transition state: an intermediate stage in the reaction from which the reaction can go in either direction. ... Catalysis is the process of increasing the rate of a chemical reaction by adding a substance Catalyzed ... 12.7: Catalysis - Chemistry LibreTexts One such reaction is catalytic hydrogenation, the process by which hydrogen is added across an alkene C=C bond to afford the saturated alkane product. A comparison of the reaction coordinate diagrams (also known as energy diagrams) for catalyzed and uncatalyzed alkene hydrogenation is shown in Figure 12.7. 1.
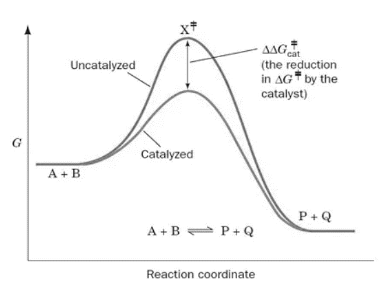
12.7 Catalysis - Chemistry 2e | OpenStax For the first step, Ea = 80 kJ for (a) and 70 kJ for (b), so diagram (b) depicts the catalyzed reaction. Activation energies for the second steps of both mechanisms are the same, 20 kJ. Homogeneous Catalysts, A homogeneous catalyst is present in the same phase as the reactants. Catalysis (12.7) - Chemistry 110 - Unizin The catalyzed reaction is the one with lesser activation energy, in this case represented by diagram b. Check Your Learning. Reaction diagrams for a chemical process with and without a catalyst are shown below. Both reactions involve a two-step mechanism with a rate-determining first step. 5.6: Reaction Energy Diagrams and Transition States In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this: Answered: Consider the following free energy… | bartleby Science Biology Q&A Library Consider the following free energy diagram for an uncatalyzed and enzyme-catalyzed reaction. Select all th statements that are true. Without enzyme With enzyme A+B Time AB O a. The rate of the enzyme catalyzed reaction is faster than the uncatalyzed reaction O b. The change in free energy for the reaction is greater ...
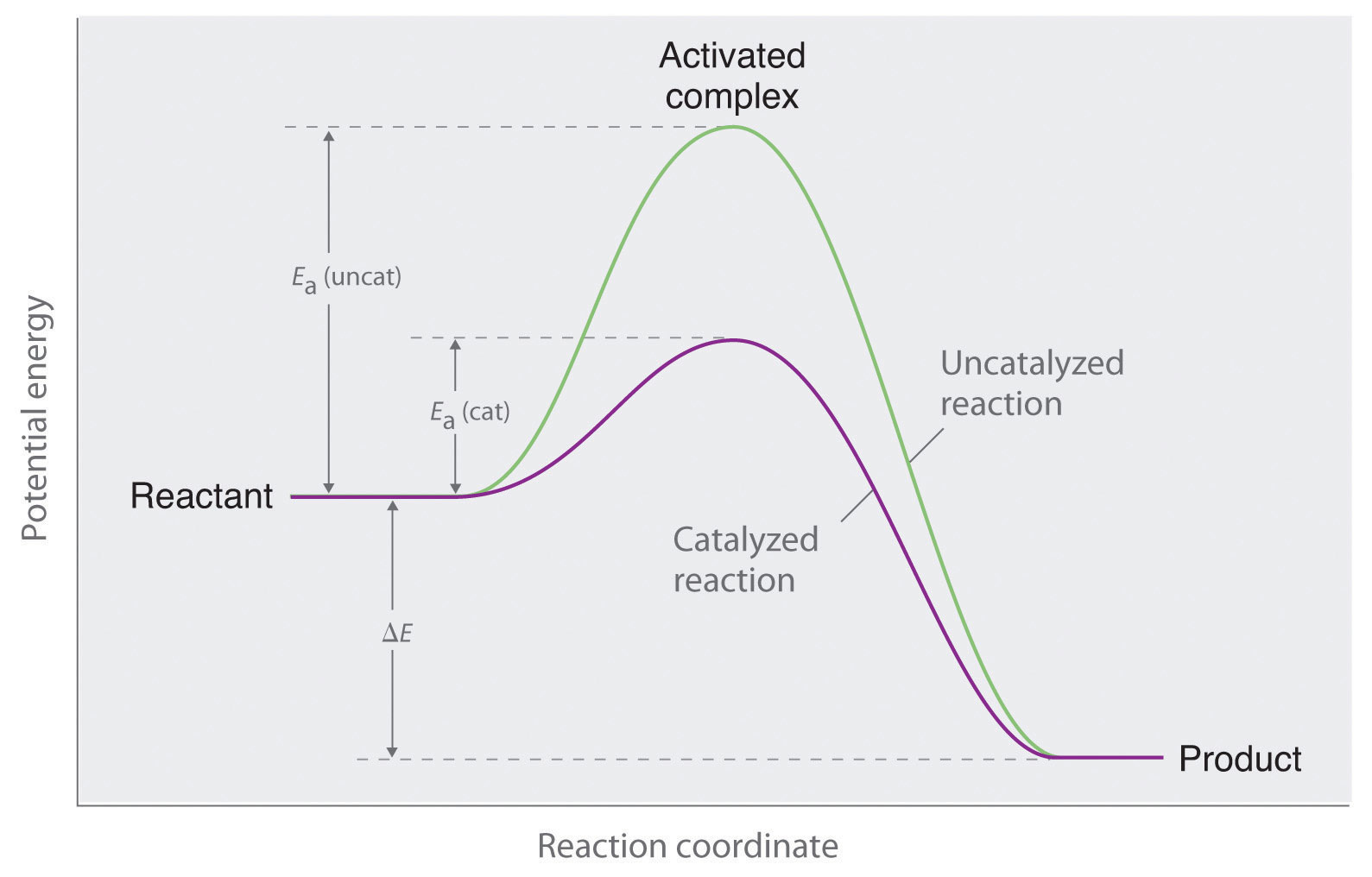
14.7: Catalysis - Chemistry LibreTexts The only effect of the catalyst is to lower the activation energy of the reaction. The catalyst does not affect the energy of the reactants or products (and thus does not affect ΔE). (CC BY-NC-SA; anonymous) The green line represents the uncatalyzed reaction. The purple line represent the catalyzed reaction . A catalyst affects Ea, not Δ E.
Solved Label the following reaction energy diagram for a - Chegg Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. Transition State Transition State Transition intermediate Reaction Intermediate Transition State Transition Uncatalyzed Transition State Reactants Products Catalyzed Ea(fwd) no catalyst Uncatalyzed AHxn < 0 Potential energy Ea(rev) no catalyst Ea(fwd) no catalyst Ea(rev) no catalyst ...
18.7: Kinetics of Catalysis - Chemistry LibreTexts Figure 18.7. 1: Lowering the Activation Energy of a Reaction by a Catalyst. This graph compares potential energy diagrams for a single-step reaction in the presence and absence of a catalyst. The only effect of the catalyst is to lower the activation energy of the reaction.
4.6 Catalysis - Inorganic Chemistry for Chemical Engineers - BCcampus For the first step, Ea = 80 kJ for (a) and 70 kJ for (b), so diagram (b) depicts the catalyzed reaction. Activation energies for the second steps of both mechanisms are the same, 20 kJ. Homogeneous Catalysts, A homogeneous catalyst is present in the same phase as the reactants.
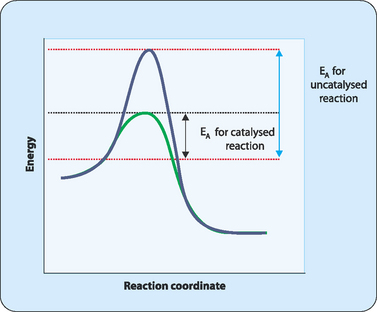
What is the difference between a catalyzed and an uncatalyzed reaction ... The addition of a catalyst to a reaction lowers the activation energy, increasing the rate of the reaction. The activation energy of the uncatalyzed reaction is shown by Ea, while the catalyzed reaction is shown by Ea'. The heat of reaction (ΔH) is unchanged by the presence of the catalyst.
Energy Changes in Catalytic Reactions - QS Study A catalyst provides a different route with lower activation energy. Fig: Energy diagram for unanalyzed and catalyzed reactions. In Figure, the unanalyzed reaction is shown as a single step with high energy of activation. The catalyzed reaction is shown as a two-stage process with lower activation energy and is, therefore, faster.
Answered: Sketch a qualitative reaction energy… | bartleby Sketch a qualitative reaction energy diagram for a chemical reaction with and without a catalyst. Assume the uncatalyzed reaction is endothermic. Note: Because the sketches are only qualitative, the energies in them don't have to be exact. They only have to have the right relationship to each other.
energy diagram catalyzed vs uncatalyzed reaction The energy diagram for a uncatalyzed reaction is an alternative way to describe a reaction. It's just a graph that shows the concentration of the reactant as well as the product. If your reaction isn't catalyzed, then the energy diagram will show this, meaning that the reaction has an exothermic reaction.
Catalysts Definition and How They Work - ThoughtCo A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. The only difference between a catalyzed reaction and an uncatalyzed reaction is that the activation energy is ...
Solved Label the following reaction energy diagram for a - Chegg Best Answer. 88% (42 ratings) Transcribed image text: Label the following reaction energy diagram for a catalyzed and an uncatalyzed process.
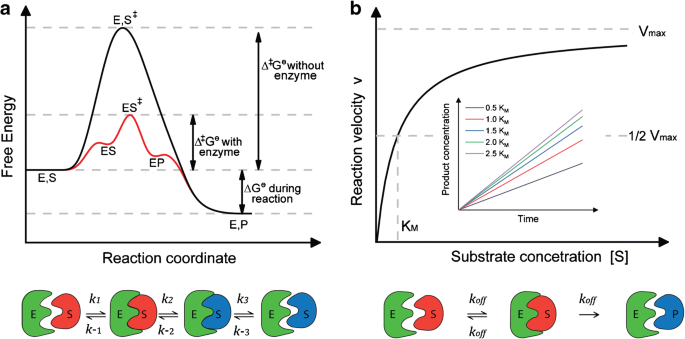
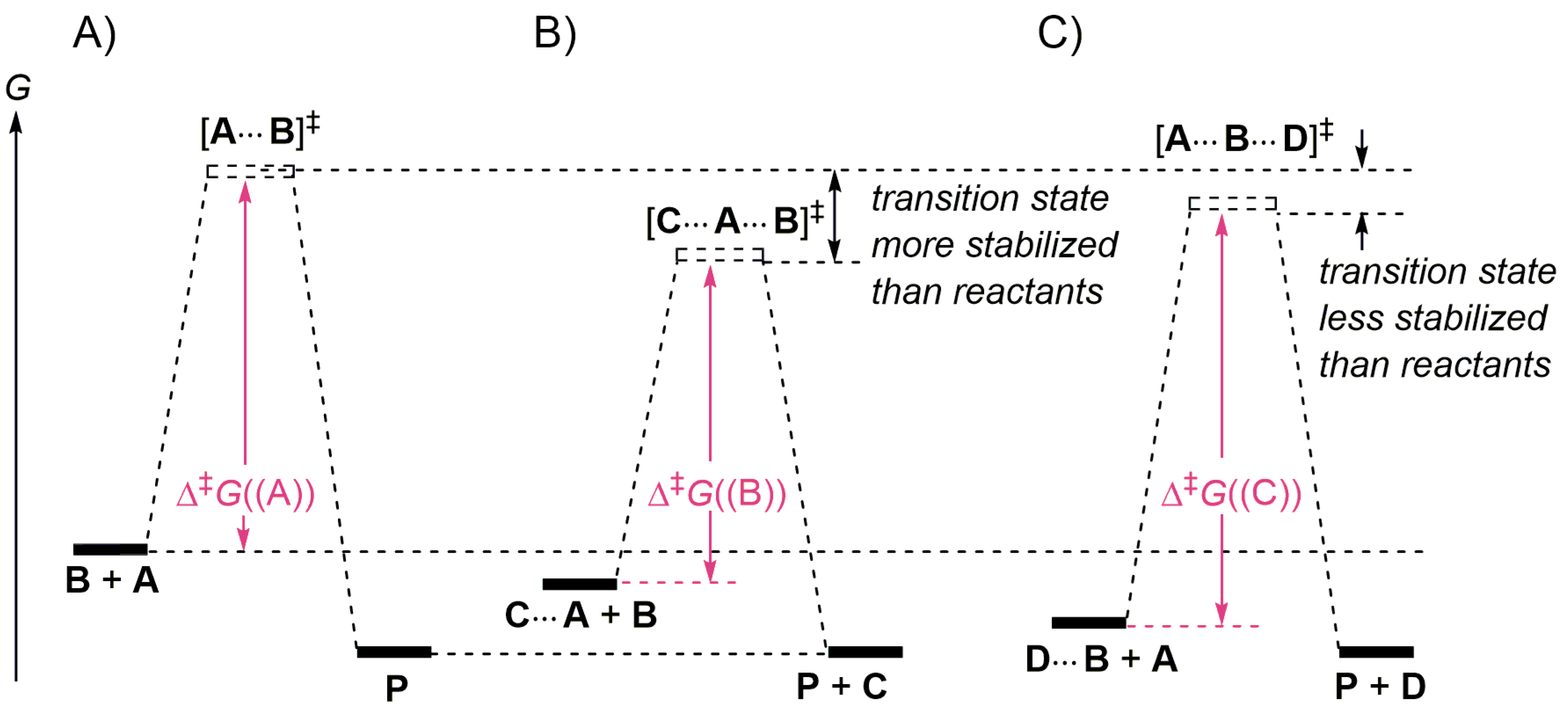
Energy diagrams for enzyme‐catalyzed reactions: Concepts and ... The energy diagram for a reaction model consisting of one enzyme, one substrate, and one product is depicted in many books where it is compared with that for the uncatalyzed reaction. The survey of several Biochemistry textbooks reveals a high diversity of profiles for the same process. A, C, and D are adapted from Refs. 5, 3, and 2, respectively.
Solved Label the following reaction energy diagram for a | Chegg.com Label the following reaction energy diagram for a catalyzed and an uncatalyzed process. AHpXn > 0 Ealfwd) no catalyst Earrev) with catalyst Reactants Earrev) no catalyst Eaſfwd) with catalyst Potential energy AHpxn < 0 Products Uncatalyzed Catalyzed Transition State Reaction Intermediate Reaction progress Reset Zoom,



















Komentar
Posting Komentar